Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Antiedemigeno Dog-Cat Slow Release’: A Potent In Vitro Inhibitor of LPS Activity
*Corresponding author:Benedetta Belà, Department of Veterinary Medicine, University of Teramo, Teramo, Italy.
Received:February 6, 2023;Published:March 03, 2023
DOI: 10.34297/AJBSR.2023.18.002438
Abstract
The present study wants to in vitro evaluate the effect of nutraceutical and chemical compounds on the adsorption, transport and permeability of endothelial cells. In particular, the research analyzed the ability of a specific nutraceutical product, ‘Antiedemigeno Dog-Cat slow release’, to alleviate and repair endothelial damage using damaged cultured S-CADMEC cells (Human Dermal Microvascular Endothelial Cells) treated with lipopolysaccharide (LPS). The results obtained from this study showed that there is a dose-response reduction of vascular permeability after the administration of the ‘Antiedemigeno Dog-Cat slow release’ respect to the cells treated with only LPS. In fact, a concentration of 0.312 mg/mL of ‘Antiedemigeno Dog-Cat slow release’ promotes a 43% reduction in vascular permeability, while a concentration of 0.156 mg/mL induces a 30% reduction. These results are very important because encourage to treat endothelial damage and related increase in vascular permeability using natural components limiting the use of drugs with several side effects.
Keywords: In Vitro Study; Endothelial Cells; Vascular Permeability; Inflammation; Lipopolysaccharide; Nutraceutical
Introduction
Endothelial tissue lines the inner surface of the blood and
lymphatic vessels, it consists of a monolayer of flat and polygonal
cells, called Endothelial cells (ECs), which come into direct contact
with the blood (or lymph) in their apical part; at the base, on the
other hand, they are anchored to the basal lamina and through it to
the underlying tissues. The endothelial cells (ECs) are very thin and
closely linked to each other and the endothelial surface does not
present any discontinuity (except for sinusoids). The endothelium
can be considered an autocrine and paracrine organ as it is able to
secrete several chemical mediators that modify cell behavior; the
result is a modulation of vascular tone and blood flow in response to
nervous, humoral and mechanical stimuli. The functions performed
by the endothelium are different:
a. Barrier function: the endothelium is like a semi-permeable
membrane that controls the passage of substances from the
extracellular fluid to the bloodstream and viceversa;
b. Regulation of coagulation, fibrinolysis and platelet
aggregation;
c. Control of adhesion and infiltration of leukocytes;
d. Control of smooth muscle cell proliferation in the media
tunic;
e. Modulation of tone, permeability and vascular structure
playing a very important role in the remodeling observed in
hypertension and re-stenosis after percutaneous coronary
intervention;
f. Formation of new blood vessels (angiogenesis).
However, the endothelium itself is the target of a variety of
neuro-hormonal signals; it also has mechanical “sensors” through
which it constantly monitors the hemodynamic forces to which
it is subjected. In response to these stimuli, the endothelial cells
release vasoactive substances whose balance maintains vascular
homeostasis. The functionality of the endothelium is so important
for the health of the whole organism; the term “endothelial
dysfunction” describes the impairment of the normal endocrineparacrine
activity of the endothelium associated with a reduction
in vasodilation capacity and appearance of pro-coagulant and
pro-inflammatory activities, atherosclerosis, hypertension, and
thrombosis. In the presence of endothelial dysfunction, therefore,
the endothelium can turn into a harmful organ as it is induced to
synthesize substances with a vasoconstrictive, pro-aggregating
and pro-inflammatory action, which represent the basic event
for the development of various cardiovascular diseases. Under
inflammatory conditions, penetration of ECs by macromolecules
and immune cells can be achieved via both transcellular and
paracellular mechanisms. When inflammation occurs, a multitude
of vasoactive cytokines, growth factors and signal modulators react
with structural components of the endothelial cells to control their
permeability. Histamine, thrombin, platelet-activating factor (PAF),
thromboxane A2 (TXA2), cytokines (Tumor Necrosis Factor-α
(TNF-α) and Interleukin-1β (IL-1β)) increase vascular permeability
inducing a dysregulation of the vascular barrier function with
plasma leakage [1]. Thrombin stimulation of cytoskeletal signaling
pathways has been shown to manipulate cell permeability [2];
while Lipopolysaccharide (LPS) induces loss of the junction barrier
and cell detachment by activating protein tyrosine kinase (PTKs)
and caspase cleavage reactions [3]. Conversely, the junctional
adhesion molecule (JAM) can reduce the permeability by initiating
cell adhesion [4] and the Angiopoietin-1 (Ang-1) can protect the
function of the endothelial barrier through the regulation of
junctional complexes [5]. Disruptions to the integrity of the barrier
and the related microvascular hyperpermeability are associated
with many systemic diseases. Pathological states of angiogenesis
include heart disease, diabetes, cancer, stroke, hypertension,
arthritis and Alzheimer’s [6, 7]. The increase of tissue permeability
can be caused by weak and hemorrhagic vessels becoming
edematous and intensify with an irregular flow of fluid through the
vessels [6]. Due to their essential role, there is immense interest in
the signaling properties of the EC junction molecules under both
physiological and pathological conditions; furthermore, expanding
the knowledge of the endothelial junction behavior and the
agents that influence it, leads to new therapies for the control of
endothelial permeability. Initially, to counteract tissue permeability
and consequent bleeding, were used drugs and chemical substances
able to alter the functionality of other organs; these drugs are used
especially in the surgical field when it’s necessary to intervene
quickly with substances that act immediately. Fortunately, in recent
years, there has been a focus on the study of substances of natural
origin and their possible effects on human/animal health. Several
plant derivatives exert functions comparable to those of a drug;
moreover, it has been seen that often the administration of the active
ingredients derived from these plants is not associated with harmful
side effects such as those caused by different types of drugs even
if this doesn’t mean that all substances of natural origin are good.
In 1989, Dr. Stephen De Felice introduced the term “Nutraceutical”
combining the words “nutrition” and “pharmaceutical” to indicate
how a natural constituents present in plants or foods can be
synthesized to create substances useful for preventing and treating
certain diseases. There has been a lot of confusion between the term
“nutraceutical” and the others such as “functional food”, dietary
supplement”, “medical food”, “pharmafood”, “phytochemical” etc.
However, nutraceuticals, standing between pharmaceuticals and
foods, have experienced challenges with safety and health claim
trials [8], in comparison to the pharmaceuticals, uni-targeted pure
compounds with high-dose use, nutraceuticals are multi-targeted
mixtures existing at low concentrations [9] comprising more
than a single food or plant component, that may be a contributing
active ingredient, but their regulation varies widely around the
world [10]. Nutraceuticals comprised herbal products, isolated
nutrients, dietary supplements, diets, genetically engineered foods
and processed products such as soups, cereals, and beverages
[11] but then, with the passage of the Dietary Supplement Health
and Education Act of 1994, was expanded to include minerals,
herbs, vitamins, and other botanicals, amino acids and any dietary
substance [12]. Nutraceutical can be classified on the bases of their
mechanism of action, chemical nature, and food bioavailability;
among the nutraceutical mechanisms of action the most important
are:
a. Anti-cancer;
b. Antioxidant;
c. Anti-inflammatory;
d. Osteogenetic or bone protective;
e. Anti-microbial;
f. Anti-aggregate;
g. Anti-hypertensive properties.
The development of major diseases is supported by oxidation processes that occur naturally in the human/animal body [13]. Gerschman, in 1954, formulated a general theory of oxygen toxicity describing the oxygen-induced damage that is caused by free radical intermediates. The oxidizing free radicals are generated in excessive amounts when the living organisms are exposed to the increased pressure of oxygen [14, 15]. Free radicals and other reactive oxygen species (ROS) are considered potentially harmful agents but are also known to produce various cellular structures and to fight pathogens [16, 17]. The antioxidant activity of nutraceuticals is affected by their chemical structure; dietary components with antioxidant functions comprise ascorbate, α-tocopherol, β-carotene, linoleic and linolenic acids, copper, manganese, zinc, selenium, and cysteine [14]. The clinical trials on the role of antioxidants have mainly focused on several compounds, such as carotenoids, vitamins C and E [16]. The ‘Antiedemigeno Dog-Cat Slow Release’ tested in the present study contains Vitamin C (L-ascorbic acid) mixed with other compounds such as pineapple and horse chestnut. It’s already known that bromelain derived from pineapple displays anti-inflammatory properties amplifying ascorbic acid effect. On the other hand, horse chestnut and its active ingredient, Escin, act at blood vessel level increasing capillary resistance and reducing their permeability. The combination of these three components could have interesting anti-inflammatory and protective effects on capillaries and blood vessels. There aren’t studies that evaluated the possible effects of a combination of these elements on humans or animals; the results obtained from the present study show how a similar combination of active ingredients can in vitro reduce an induced vascular permeability.
Materials and Methods
Product Features
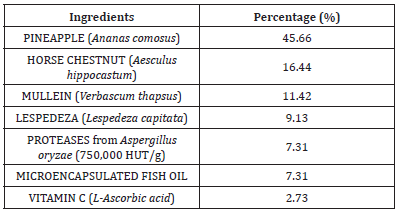
Table 1 shows the components (expressed as a percentage) of the product ‘ANTIEDEMIGENO DOG-CAT SLOW RELEASE’ tested in this study; it mainly consists of Ananas comosus (45.66%), Aesculus hippocastum (16.44%), Verbascum thapsus (11.42%), Lespedeza capitata (9.13%), proteases from Aspergillus oryzae (7.31%), microencapsulated fish oil (7.31%) and L- ascorbic acid (2.73%).
Experimental Procedure
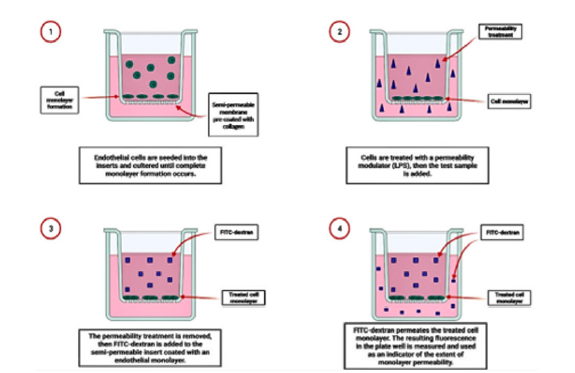
Times and concentrations used in the present study were determined by preliminary experiments. The assay allows the evaluation of chemical and pharmacological compound effects on endothelial cell adsorption, transport and permeability. The assay was performed on S-CADMEC cell line (Human Dermal Microvascular Endothelial Cells; Cell Applications, Inc., San Diego, CA); it was conducted in triplicate, using a 24-well plastic plate containing 24 collagen-coated inserts. Each insert consists of a membrane containing 1 μm pores to permit apical and basolateral access of cells to media and molecules of interest. Endothelial cells are seeded onto the inserts forming a monolayer and incubated occluding the membrane pores. The cell monolayer was first treated with LPS solution at a concentration of 100 ng/mL for 6 h to increase the permeability of the endothelial monolayer. Subsequently, cells were incubated for 24h with the sample at concentrations of 0.312, 0.156, 0.078 mg/mL and 150 μL of FITC-Dextran working solution were added to each insert. The plate was incubated, away from light, for 20 minutes at room temperature.
The procedure is schematized in (Figure 1).
Subsequently, 100 μL of media from each well were transferred to the black 96-well opaque plate wells for fluorescence measurement. The plate was read using a fluorescence plate reader with appropriate filters for 485 nm and 535 nm (excitation and emission respectively). After completion of FITC-Dextran permeability testing, the endothelial monolayer was stained for brightfield imaging of monolayer integrity. The media was carefully removed from each insert and were added 150 μL of cell stain, then, the plate was incubated at room temperature for 20 minutes. Each insert was carefully rinsed twice with 200 μL of PBS. The insert may be left in the second rinse during microscopic (brightfield) imaging. Fluorescence intensities were quantified (one second counting time) on a Synergy H1 HYBRID Multi-Mode Reader (BioTek Instruments) using Gen5 software (BioTek). The “C-” sample exhibited a visually confluent monolayer, as supported by the finding of low FITC-Dextran permeability (a “positive control” for monolayer integrity). Disruption of monolayer integrity was observed both visually and by quantification of increased plate well solution fluorescence following LPS treatment. After damage induced by LPS (100 ng/ml) for 6 h, the quantification of the fluorescence intensity of the cells treated for 24 h with the sample “Antiedemigeno” at the concentrations of 0.312, 0.156 and 0.078 mg/ml showed a dose-response reduction of vascular permeability compared to the cells treated only with LPS.
Lipopolysaccharide (LPS) is one of the components of the
outer cell membrane of Gram-negative bacteria such as Salmonella,
Escherichia coli and Helicobacter [8]. It is found in the outer leaflet
of the outer membrane of this class of bacteria, while the inner
leaflet is made up of phospholipids. It is a large molecule consisting
of a lipid portion and a polysaccharide able to induce a strong
immune response in animals.
LPS are made up of three main regions:
a) Lipid A or glycolipid portion;
b) an oligosaccharide core;
c) the O antigen, a polysaccharide chain consisting of 20-50
repetitive units that generally can contain up to eight sugars.
Specifically, lipid A is formed by a glucosamine dimer which binds (through ester and amide bonds) fatty acid chains of different lengths, improving their anchoring to the external membrane of bacterium; the most common are caproic, lauric, palmitic, myristic and stearic acid. Its glycolipid nature allows it to anchor itself in the outer membrane of the bacteria, while the polysaccharide portion of the LPS protrudes outside the membrane. Lipid A represents the bacterial endotoxin which carries out the toxic action on the organism; it’s very important because its structural variation can modify the stimulatory activity of LPS on the innate immune system [18]. The oligosaccharide core consists of approximately 9-12 saccharide units; it often contains a heptose sugar, ethanolamine and 2-keto-3-deoxyottonic acid (KDO); this is the hydrophilic portion of the molecule (together with the side chain). Finally, the O antigen (O-Specific Chain), also called side chain, consists of a repeat of 20-50 units; it represents the outermost portion and, therefore, the one exposed to the immune system. Its composition differs depending on the species and can also be different within the species, thus characterizing the different strains. The O-specific antigen of lipopolysaccharide is also responsible for an intense response by the organism, with fever and shock due to the increase in vascular permeability and vasodilation. During the bacterial replication, small amount of LPS can be released by the fragmentation of the bacterial surface while, greater quantities of Lipopolysaccharide are released into the circulation following bacterial death. Recent studies showed that the presence of these fragments is related with several chronic inflammatory diseases like cardiovascular, neurodegenerative, and metabolic diseases as LPS can induce a constant inflammatory state [18]. For all these reasons the LPS was used to create damage on the endothelial cell line.
Results
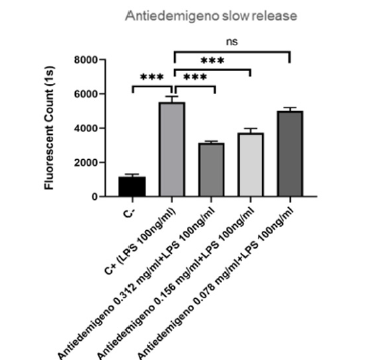
As we can see at the Figure 2, the ‘Antiedemigeno Dog-Cat slow release’ is able to promote a reduction in vascular permeability; the degree of permeability is determined by measuring the fluorescence (directly proportional to vascular permeability) through a plate reader.
The best result was obtained by the addition of 0.312 mg/mL of ‘Antiedemigeno slow release’ inducing a great decrease in the fluorescent count respect to the sample treated with only LPS, corresponding to the column ‘C+LPS’ (Figure 2). It is very interesting to note that even lower concentration of the product (0.156 mg/ mL) can exert modest anti-edema effects (Figure 2); while the addition of 0.078 mg/mL of Antiedemigeno showed a very small effect on the reduction of endothelial permeability (Figure 2).
Discussion
The term nutraceutical, later associated with nutraceutical products, was introduced in 1989 by Dr. Stephen De Felice, who coined this definition by combining the words “nutrition” and “pharmaceutical”, to indicate how the natural constituents present in foods or plants can be synthesized to create drugs that are useful for preventing and treating certain diseases. Nutraceutical products, in our system, are classified as food supplements and are mostly associated with so-called functional foods: products that, in addition to having a nutritional effect, have added substances that can influence some physiological processes of the body. Nutraceuticals perform the specific function of improving the health status preventing the risk of disease. They are substances derived from plants, microbial agents and foods that can be integrated into a diet either through the intake of functional foods or in the form of supplements in tablets or capsules. In the present study was tested the ability of a specific nutraceutical product, ‘Antiedemigeno Dog-Cat slow release’, to counteract vascular permeability induced by LPS. The product consists of a mix of natural compounds with known beneficial properties, the main components was the Ananas comosus (45.66%) containing a sulfhydryl proteolytic enzyme (bromelain) that is a promising compound of plant origin used in many branches of medicine. In fact, bromelain’s immunomodulatory and anti-inflammatory properties and its effect on the production/activity of cytokines involved in the inflammatory process, was the topic of numerous studies. Using in vitro research models was observed that bromelain inhibits the activity of cytokines during inflammatory induction by lipopolysaccharide (LPS) [19-21]. LPS induces the activation of several intracellular signaling pathways, including the Tumor Necrosis Factor-alpha (TNF-α), Nuclear Factor of activated B cells (NF-κB) and several mitogen-activated protein kinase (MAPK) pathways. Hou and Huang et al. [21] showed that bromelain caused significant suppression of Interleukin 6 (IL-6), Interleukin 1β (IL- 1β) and Tumor Necrosis Factor α (TNF-α) production in a dosedependent manner. In addition, bromelain is also able to inhibit both NF-κB transcription factor and MAPK pathways, decreasing cyclooxygenase-2 (COX-2), PGE2 mRNA levels and extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and p38 activation; a similar anti-inflammatory effect of bromelain has also been observed in rat primary microglial cells [22], human U937 macrophages [20] and RAW 264.7 cells [23]. Moreover, bromelain exhibits anti-inflammatory properties in macrophages by inhibiting the expression of essential pro-inflammatory cytokines and chemokines including macrophage inflammatory protein 1alpha (MIP-1α), macrophage inflammatory protein-1beta (MIP-1β), monocyte chemoattractant protein-1 (MCP-1), IL-8, IL- 1β IL-6 and the cyclooxygenase pathway [22, 24]. Habashi, et al. [22] displayed how a bromelain treatment can reduce the expression of inducible nitric oxide synthase (iNOS). This enzyme is very important because synthesizes the nitric oxide (NO), an intercellular signaling molecule involved in the regulation of vasomotor tone, cell adhesion, inhibition of platelet aggregation and vascular smooth muscle cell proliferation. An excessive iNOS activity is observed in cancer [25] and autoimmune diseases [26]. Pro-inflammatory cytokines are closely related to an inflammatory process, among them, IL-exerts several physiological functions in acute and chronic inflammation [19]. On the other hand, Tumor Necrosis Factor α (TNF-α) can regulate immune responses by activation of TNF receptors and related pathways like NF-κB and MAPKs. After phosphorylation, NF-κB can bind a specific promoter region of pro-inflammatory genes and the upregulation of these genes leads to have a release of pathogenic mediators involved in the establishment of inflammation. Bromelain displays a protease activity able to reduce the amount of pro-inflammatory cytokines released by LPS activity; an interesting factor is that stem-derived bromelain seems to contain more proteases respect the fruitderived bromelain. Bromelain proteolytic action is the main responsible for its pharmacological activities [19]. Although the mechanisms of action are not yet fully understood, it has long been known that bromelain exerts an anti-inflammatory action by suppressing the action of LPS. The second most present component of the ‘Antiedemigeno Dog-Cat slow release’ is Aesculus hippocastanum; its active compound, Escin, is used as a traditional medicine for centuries [27] and it’s a plant compound obtained from the mixture of saponins contained in the seeds and leaves of the horse chestnut. Escin is still used to treat certain conditions, including hemorrhoids [28], varicose veins, hematoma and venous congestion [29]. It was first isolated in 1953 [27] and has demonstrated anti-edematous, anti-inflammatory and venotonic properties in various preparations [30]. Escin can reduce edema and increase venous tone, producing measurable improvements in venous hemodynamics. Escin is also able to decrease the levels of pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6 in LPSinduced macrophages by decreasing the HMGB1 (High Mobility Group Box 1) release. HMGB1 is found in large quantities within the nucleus of all eukaryotic cells and its main role is to remodel chromatin. Furthermore, it has recently been discovered that this protein is an important mediator in the inflammatory process, especially in the case of cell necrosis. Therefore, HMGB plays an important role in triggering inflammation, but it would also appear to be involved in innate and adaptive responses and in the repair of tissue damage. It’s secreted by stressed cells; in particular, HMGB1 passes from the nucleus to the cytoplasm and is then secreted through lysosomes and directly into the extracellular space. A reduction in its production can be related to an improvement in the state of stress. Pre- and post-treatment with Escin displays antiinflammatory effects ameliorating endotoxin induces injury [31]. Another important component of the product tested in this study was Vitamin C. Scurvy is one of the oldest human diseases, the origin was clearly identified only in 1932 and today we know that it is linked to a severe deficiency of ascorbic acid (Vitamin C). This disease is characterized by multiple signs and symptoms including hemorrhages and anemia highlighting how Vitamin C is involved in the regulation of vascular processes. In fact, hemorrhages are the consequence of increased permeability of blood vessels and Vitamin C is essential for the formation of connective tissue, which provides the vascular wall with its strength and flexibility. Vitamin C is also directly involved in the health of the immune system: its proven antioxidant power has an important role in controlling inflammation; several evidence demonstrate other functions more or less directly related to the defense against external pathogens like: enhancement of phagocytic activity and collagen production, maintenance of epithelial barrier integrity, promotion of lymphocyte replication and wound resolution. Furthermore, Vitamin C is also able to ameliorate the oxidative imbalance and vascular remodeling induced by different stressors, including reperfusion injury following myocardial infarction [32], hyperoxya [33], shear stress [34], prolonged immobilization [35], glucose load [36] and mental stress [37]. During a physical activity it’s able to increase skeletal muscle blood flow and oxygen consumption via local vasodilation [38]. Vitamin C attenuates the endothelial barrier permeability [39], an aspect that has major implications in infectious disorders [40], which are also known to cause a systemic surge in oxidative stress [41]. Therefore, the antioxidant roles of Vitamin C and its protective effects on endothelial permeability are fundamental during infective processes [42]. Vitamin C is also able to prevent lipopolysaccharide damage, in fact, while LPS promotes a reduction of superoxide dismutase (SOD) levels increasing the malondialdehyde, Vitamin C pretreatment restores the normal levels of SOD decreasing the amount of malondialdehyde. Malondialdehyde is naturally present in tissues, where it is a manifestation of oxidative stress; its reduction indicates an improvement in the inflammatory state [43]. Furthermore, Vitamin C has also been shown to improve the effects of other agents in a synergistic manner: when added to metformin, it reduces cardiovascular diabetic complications [44], when added to glucagon-like peptide 1 (GLP–1) it further ameliorates endothelial function and reduces oxidative stress in Type 1 diabetic patients [45]. Specific effects of Verbascum thapsus and Lespedeza capitata on the endothelial permeability are not yet present in literature, thus, it is possible that the association of these two compounds with the others present in the product like Ananas comosus (bromelain), Aesculus hippocastum (escin) and Vitamin C play a beneficial action all together empowering each other, in fact, has already been proven that natural products with different mechanisms of action may have synergetic effects. As can be seen from the results, the most effective concentration of the product is that of 0.312 mg/mL causing a 43% reduction in vascular permeability, followed by the dose of 0.156 mg/mL corresponding to a 30% reduction in permeability. The lower concentration of the product was, however, able to induce a slight decrease in vascular permeability of about 9%. The results displayed a dose-response reduction of endothelial permeability.
Conclusion
Following the damage induced to endothelial cells with LPS (100 ng/mL) for 6 hours and the subsequent treatment for 24 hours with the ‘Antiedemigeno Dog-Cat Slow Release’ at concentrations of 0.312, 0.156 and 0.078 mg/mL, was observed a dose-response reduction of vascular permeability by 43%, 30% and 9% respectively, in comparison to cells treated with LPS only. In addition to being positive, these results are very encouraging to treat endothelial damage and related increase in vascular permeability using natural origin components and limiting the use of drugs often with several side effects. It is well known that an increased vascular permeability is associated with a series of events that lead to severe inflammatory states often associated with important pathologies; the fact that the product under study can reduce it, limits the subsequent onset of more complex clinical conditions.
References
- Yamamoto K, Takagi Y, Ando K, Fukuhara S (2021) Rap1 Small GTPase Regulates Vascular Endothelial-Cadherin-Mediated Endothelial Cell–Cell Junctions and Vascular Permeability. Biol Pharm Bull 44(10): 1371-1379.
- Vouret Craviari V (1998) Regulation of the actin cytoskeleton by thrombin in human endothelial cells: role of Rho proteins in endothelial barrier function. Mol Biol Cell 9(9): 2639-2653.
- Bannerman DD, M Sathyamoorthy, SE Goldblum (1998) Bacterial lipopolysaccharide disrupts endothelial monolayer integrity and survival signaling events through caspase cleavage of adherens junction proteins. J Biol Chem 273(52): 35371-35380.
- Bazzoni G, OM Martinez Estrada, F Orsenigo, M Cordenonsi, S Citi, et al. (2000) Interaction of junctional adhesion molecule with the tight junction components ZO-1, cingulin and occludin. J Biol Chem 275(27) :20520-20526.
- Li X, Milena Stankovic, Claudine SB, Christopher NH, Michelle Parsons, et al. (2008) Basal and angiopoietin-1-mediated endothelial permeability is regulated by sphingosine kinase-1. Blood 111(7): 3489-3497.
- Bates DO, N J Hillman, B Williams, C R Neal, T M Pocock (2002) Regulation of microvascular permeability by vascular endothelial growth factors. J Anat 200(6): 581-597.
- Mooradian AD (1998) Effect of aging on the blood-brain barrier. Neurobiol Aging 9(1): 31- 39.
- Shekhar V, Jha AK, Dangi JS (2014) Nutraceuticals: A Re-emerging Health Aid. Int Conference on Food Biol Med Sci: pp 105-107.
- Shahidi F (2012) Nutraceuticals, functional foods and dietary supplements in health and disease. J Food Drug Anal 20(1): 226-230.
- Molinos M, Carvalho V, Silva DM, Gama FM (2012) Development of a Hybrid Dextrin Hydrogel Encapsulating Dextrin Nanogel As Protein Delivery System. Biomacromolecules 13(2): 517-527.
- Andlauer W, Fürst P (2002) Nutraceuticals: A piece of history, present status and outlook. Food Res Int 35(2-3): 171-176.
- Singh UK, Deshmukh SN (2016) Nutraceuticals. MIT Int J Pharm Sci 2: 43-52.
- Bartosz G (2014) Food Oxidants and Antioxidants Chemical, Biological and Functional Properties. CRC Press Taylor Francis 236: USA.
- Gilbert DL (1981) Oxygen and Living Processes.1st edn Springer: USA.
- Gerschman R, Gilbert DL, Nye SW, Dwyer P, Fenn WO (1954) Oxygen Poisoning and X-irradiation: A Mechanism in Common. Science 119(3097): 623-626.
- Palmer HJ, Paulson KE (1997) Reactive Oxygen Species and Antioxidants in Signal Transduction and Gene Expression. Nutr Rev 55: 353-361.
- Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, et al. (2017) Oxidative Stress: Harms and Benefits for Human Health. Oxid Med Cell Longev: 8416763.
- Page MJ, Kell DB, Pretorius E (2022) The Role of Lipopolysaccharide-Induced Cell Signalling in Chronic Inflammation. Review. Chronic Stress 6: 1-18.
- Insuan O, Janchai P, Thongchuai B, Chaiwongsa R, Khamchun S, et al. (2021) Anti-Inflammatory Effect of Pineapple Rhizome Bromelain through Downregulation of the NF-B- and MAPKs-Signaling Pathways in Lipopolysaccharide (LPS)-Stimulated RAW264.7 Cells. Curr Issues Mol Biol 43: 93-106.
- Kasemsuk T, Vivithanaporn P, Unchern S (2018) Anti-inflammatory Effects of Bromelain in Lps-induced Human U937 Macrophages. Chiang Mai J Sci 45: 299-307.
- Huang JR, Wu CC, Hou RCW, Jeng KC (2008) Bromelain Inhibits Lipopolysaccharide-Induced Cytokine Production in Human THP-1 Monocytes via the Removal of CD14. Immunol Investig 37: 263-277.
- Habashi SA, Sabouni F, Moghimi A, Majd SA (2015) Modulation of Lipopolysaccharide Stimulated Nuclear Factor kappa B Mediated iNOS/NO Production by Bromelain in Rat Primary Microglial Cells. Iran Biomed J 20: 33-40.
- Lee JH, Lee JB, Lee JT, Park HR, Kim JB, et al. (2018) Medicinal Effects of Bromelain (Ananas comosus) Targeting Oral Environment as an Anti-oxidant and Anti-inflammatory Agent. J Food Nutr Res 6: 773-784.
- Hou RCW, Chen YS, Huang JR, Jeng KCG (2006) Cross-Linked Bromelain Inhibits Lipopolysaccharide-Induced Cytokine Production Involving Cellular Signaling Suppression in Rats. J Agric Food Chem 54: 2193-2198.
- Somasundaram V, Basudhar D, Bharadwaj G, No JH, Ridnour LA, et al. (2019) Molecular Mechanisms of Nitric Oxide in Cancer Progression, Signal Transduction, and Metabolism. Antioxid Redox Signal 30: 1124-1143.
- Soufli I, Toumi R, Rafa H, Touil Boukoffa C (2016) Overview of cytokines and nitric oxide involvement in immuno-pathogenesis of inflamatory bowel diseases. World J Gastrointest Pharmacol Ther 7: 353-360.
- ombardelli E, Morazzoni P, Griffini A (1996) Aesculus hippocastanum. L Fitoterapia 67(6): 483-511.
- Chauhan R, Ruby K, Dwivedi J (2012) Golden herbs used in piles treat- ment: a concise report. Int J Drug Dev Res 4(4): 50-68.
- European Medicines Agency (2009) Assessment report on Aesculus Hipoocastanum L.
- Sirtori CR (2001) Aescin: pharmacology, pharmacokinetics and therapeutic profile. Pharmacol Res 44(3): 183-193.
- Cheng Y, Wang H, Mao M, Liang C, Zhang Y, et al. (2015) Escin Increases the Survival Rate of LPSInduced Septic Mice Through Inhibition of HMGB1 Release from Macrophages. Cell Physiol Biochem 36: 1577-1586.
- Nicolás Valls, Juan G Gormaz, Rubén Aguayo, Jaime González, Roberto Brito, et al. (2016) Amelioration of persistent left ventricular function impairment through increased plasma ascorbate levels following myocardial infarction. Redox Rep 21: 75-83.
- Sushant M Ranadive, Michael J Joyner, Branton G Walker, Jennifer L Taylor, Darren P Casey, et al. (2014) Effect of vitamin C on hyperoxia-induced vasoconstriction in exercising skeletal muscle. J. Appl Physiol 117: 1207-1211.
- Blair D Johnson, Kieren J Mather, Sean C Newcomer, Timothy D Mickleborough, Janet P Wallace, et al, (2013) Vitamin C prevents the acute decline of flow-mediated dilation after altered shear rate patterns. Appl Physiol. Nutr Metab 38: 268-274.
- Thosar SS, Bielko SL, Wiggins CC, Klaunig JE, Mather KJ, Wallace JP. (2015) Antioxidant vitamin C prevents decline in endothelial function during sitting. Med Sci Monit 21: 1015-1021.
- Sergio De Marchi, Manlio Prior, Anna Rigoni, Sara Zecchetto, Fanny Rulfo, Enrico Arosio. (2012) Ascorbic acid prevents vascular dysfunction induced by oral glucose load in healthy subjects. Eur J Intern Med 23 : 54-57.
- G M S Batista, H N M Rocha, A S Storch, V P Garcia, G F Teixeira, et al. (2020) Ascorbic acid inhibits vascular remodeling induced by mental stress in overweight/obese men. Life Sci 250 : 117554.
- Richards JC, Crecelius AR, Larson DG, Dinenno FA. (2015) Acute ascorbic acid ingestion increases skeletal muscle blood flow and oxygen consumption via local vasodilation during graded handgrip exercise in older adults. Am J Physiol Heart Circ Physiol 309: H360-H368.
- Meredith ME, Qu ZC, May JM, (2014) Ascorbate reverses high glucose- and RAGE-induced leak of the endothelial permeability barrier. Biochem. Biophys Res Commun 445, 30-35.
- Zhao Li, Mingzhu Yin, Haifeng Zhang, Weiming Ni, Richard W Pierce, et al. (2020) BMX Represses Thrombin-PAR1-Mediated Endothelial Permeability and Vascular Leakage During Early Sepsis. Circ Res 126: 471-485.
- Mironova GD, Belosludtseva NV, Ananyan MA, (2020) Prospects for the use of regulators of oxidative stress in the comprehensive treatment of the novel Coronavirus Disease 2019 (COVID-19) and its complications. Eur Rev Med Pharmacol Sci 24: 8585-8591.
- Carr AC, Rowe S, (2020) The Emerging Role of Vitamin C in the Prevention and Treatment of COVID-19. Nutrients 12: 3286.
- Shati AA, Zaki MSA, Alqahtani YA, Al-Qahtani SM, Haidara MA, Dawood AF et al. (2022) Antioxidant Activity of Vitamin C against LPS-Induced Septic Cardiomyopathy by Down-Regulation of Oxidative Stress and Inflammation. Curr Issues Mo. Biol 44: 2387-2400.
- Gillani SW, Sulaiman SAS, Abdul MIM, Baig MR. (2017) Combined effect of metformin with ascorbic acid versus acetyl salicylic acid on diabetes-related cardiovascular complication; a 12-month single blind multicenter randomized control trial. Cardiovasc Diabetol 16: 103.
- Antonio Ceriello, Anna Novials, Emilio Ortega, Silvia Canivell, Lucia La Salaet, et al. (2013) Vitamin C further improves the protective effect of glucagon-like peptide-1 on acute hypoglycemia-induced oxidative stress, inflammation, and endothelial dysfunction in type 1 diabetes. Diabetes Care 36: 4104-4108.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.